1-(Chloromethyl)naphthalene | CAS: 86-52-2
| Name | 1-Chloromethyl naphthalene |
| CAS | 86-52-2 |
| Appearance | White prismatic crystals |
| Molecular Formula | C11H9Cl |
| Molecular Weight | 176.64 |
| Assay | 99% |
| Packing, Delivery | 25kg/drum; Delivery within 3-5 working days |
| Sample | Available |
| Payment | L/C, D/A, D/P, T/T, Other |
ORIENTRED is a leading manufacturer and supplier of 1-(Chloromethyl)naphthalene (CAS: 86-52-2) in China. It focuses on producing 1-chloromethyl naphthalene with high purity/assay.
- 1-Chloromethyl naphthalene is of good quality and at a cheap price, and is produced in strict compliance with ISO9001, cGMP and other standards.
- We provide free samples, trial orders, bulk purchases of tons of 1-Chloromethyl naphthalene, procurement tiered pricing, and cost reduction 15%+.
- Open third-party testing and fast logistics services
Contact us now to get your exclusive quotation and COA sample.
1-(Chloromethyl)naphthalene Basic Information, Physicochemical Properties, etc.
| Basic Information | |
| Name | 1-(Chloromethyl)naphthalene |
| Synonyms | 1-(chloromethyl)-naphthalen;1-(Chloromethyl)naphthene;AKOS BBS-00004040;1-chloromethyl-naphthalen;1-Menaphthyl chloride;1-menaphthylchloride;1-(CHLOROMETHYL)NAPHTHALENE;1-CHLOROMETHYLNAPHTHYL |
| CAS Number | 86-52-2 |
| Molecular Formula | C11H9Cl |
| Molecular Weight | 176.64 |
| EINECS Number | 201-678-2 |
| Structural Formula | 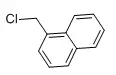 |
| Physicochemical Properties | |
| Melting Point | 32 °C(lit.) |
| Boiling Point | 291 °C |
| Density | 1.18 g/mL at 25 °C(lit.) |
| Refractive Index | n20/D 1.635(lit.) |
| Solubility | Soluble in chloroform, ethyl acetate (slightly soluble), methanol (slightly soluble) |
| Water Solubility | Insoluble in water. |
| Form | Prismatic crystals |
| Color | White |
| Sensitivity | Moisture Sensitive |
| Flash Point | >230 °F |
| Safety and Regulatory Information | |
| Merck | |
| BRN | 636885 |
| NIST Chemical Substance Information | 1-Chloro Methyl Naphthalene | CAS: 86-52-2 |
| EPA Chemical Substance Information | Naphthalene, 1-(chloromethyl) | CAS: 86-52-2 |
| Other Information | |
| Detection Methods | |
1-(Chloromethyl)naphthalene Application, Use, Usage, Synthesis Method, etc.
- Used in synthetic resins, medicines, etc.
- Organic synthesis intermediate, used to synthesize 1-naphthaldehyde.
- 1-Chloromethylnaphthalene can be used as a catalyst to prepare some important compounds, such as α-naphthyl acetic acid, by carbonylation reaction.
- 1-Chloromethylnaphthalene is an important organic synthesis raw material. It can be used as a starting material to prepare naphthyl acetic acid, 1-naphthylmethoxyacetic acid, N-methyl-1-naphthylmethylamine, etc.
Production method
The synthesis routes of 1-chloromethylnaphthalene mainly include the 1-methylnaphthalene side chain chlorination method and the naphthalene triacid method.
Naphthalene triacid method
Prepared by chloromethylation of naphthalene.
Heat naphthalene, polyoxymethylene, glacial acetic acid, 85% phosphoric acid, and concentrated hydrochloric acid to 85℃ and stir vigorously for 6h.
After the reaction is completed, cool to 15℃ and wash with water. Finally, wash with 10% potassium carbonate solution, separate the water layer, add ether, and dry with anhydrous sodium carbonate.
Then, the ether is evaporated under normal pressure, the residue is distilled under reduced pressure, and the fraction at 128-135℃ (0.67kPa) is collected to obtain the finished product.
1-Methylnaphthalene side chain chlorination method
Mix naphthalene, polyformaldehyde, Lewis acid, phase transfer catalyst, and hydrochloric acid solution with a mass concentration of 42% to 43% evenly, keep warm for reaction, stand for separation, wash the oil phase, and obtain crude 1-chloromethylnaphthalene.
Add the crude 1-chloromethylnaphthalene to an alcohol solvent, heat up to dissolve, cool down to crystallize, filter, wash, and dry to obtain a white crystalline 1-chloromethylnaphthalene product.
Blank chloromethylation reaction.
That is, under the action of anhydrous zinc chloride, the aromatic compound naphthalene reacts with formaldehyde and hydrogen chloride to produce monochloromethylnaphthalene.
Add a certain amount of naphthalene, polyformaldehyde, anhydrous zinc chloride, and 37% hydrochloric acid aqueous solution into a three-necked flask with condensation reflux, and stir and react at 60℃ for 3 hours.
Extract and separate with anhydrous ether, wash the upper oil phase with weak alkaline solution until the pH value is 7.
Evaporate to remove cyclohexane, distill under reduced pressure, and collect 1-chloromethylnaphthalene monomer.
1-(Chloromethyl)naphthalene Packaging, Storage, Delivery, Transportation, etc.
Packing: 200kg /drum or Customer specified packaging
Storage: Keep in a dark place, sealed in a dry at Room Temperature
Delivery: delivery within 3- 5 working days
Transportation: According to your needs
FAQ About 1-(Chloromethyl)naphthalene
ORIENTRED is a leading manufacturer and supplier of [Product Name] (CAS No.: XXX) in China, focusing on producing [Product Name] with high purity/content.
Where can I buy naphthalene, 1-(chloromethyl)? Price and minimum order quantity?
Answer:
Purchase through chemical suppliers (such as Sigma-Aldrich, TCI, or ORIENTRED) or pharmaceutical intermediate companies.
Please feel free to contact us for the latest quotes.
The minimum order quantity for laboratory grade is usually 1 gram.
How long is the shelf life?
Answer:
1-Chloro Methyl Naphthalene can be stored for 2 years when sealed and protected from light. It is recommended to use it up within 1 year after opening (check regularly for discoloration or agglomeration).
Is 1-Chloro Methyl Naphthalene toxic?
The concentration that can be reached in the air does not produce acute toxic effects.
When injected intraperitoneally, the acute poisoning signs of rats are: weakness, ataxia, difficulty breathing, and hypothermia.
When animals are chronically poisoned, slow development, accelerated breathing, increased oxygen consumption, higher nervous activity and hemodynamic disorders are seen.
Is Naphthalene, 1-(chloromethyl) flammable?
It is flammable when exposed to open flames and high temperatures.
It emits toxic and irritating smoke when burning.
It can react violently with strong oxidants such as chromic anhydride, chlorate, and potassium permanganate, causing combustion or explosion.






