There are several methods for the preparation and production of 1H-1,2,3-triazole. In this article, we have compiled and organized eight methods for the preparation and production of 1H-1,2,3-triazole.
Now, let ORIENTRED introduce each method in detail. Each method includes the amount of raw materials, reaction conditions and detailed operation steps.
Method 1
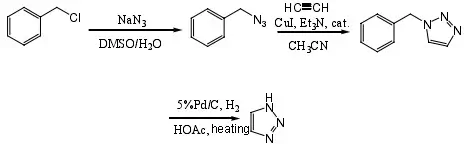
1-Benzyl-1,2,3-triazole intermediate was prepared by using benzyl chloride, sodium azide and acetylene gas as raw materials and copper-catalyzed cycloaddition as the key reaction.
The intermediate was synthesized into 1H-1,2,3-triazole under palladium/carbon catalytic hydrogenation with a total yield of 70%.
The reaction conditions of this method are mild and the emission of three wastes is small.
Operation steps
(1) Synthesis of benzyl azide
Weigh 12.6 g (0.1 mol) of benzyl chloride in a 250 mL single-necked round-bottom flask, and then add 100 mL DMSO and 20 mL water.
Add a stirrer and set up an external water bath.
Start stirring, add 13.0 g (0.2 mol) of NaN3, and stir at room temperature for 24 h.
After the reaction is completed, the reaction mixture is extracted with petroleum ether (30 mL × 4), the organic phases are combined, and then the organic phases are washed twice with 50 mL of water and once with 30 mL of saturated brine.
Dry over anhydrous Na2SO4, filter to remove the desiccant, and evaporate the petroleum ether on a rotary evaporator to obtain 12.1 g of colorless oily liquid with a yield of 91%.
The product can be used in the next reaction without any purification.
(2) Synthesis of 1-benzyl-1,2,3-triazole
Weigh 6.65 g (0.05 mol) of benzyl azide into a 100 mL ground-mouth round-bottom flask, then add 2.02 g Et3N (0.02 mmol), 50 mL acetonitrile and 0.95 g CuI (0.005 mol) in sequence, put in a stirrer, and install a three-way valve.
Expel the air from the system with a nitrogen flow through the three-way valve, then connect the three-way valve to an acetylene bag, replace the nitrogen with acetylene gas, and stir at room temperature for 24 h.
After the reaction is completed, filter the filtrate and remove the solvent on a rotary evaporator, then pour 20 ml of ether again, filter with a sand core funnel, and remove the solvent from the light yellow filtrate under reduced pressure to obtain 7.08 g of the title compound with a yield of 89%. It can be directly used in the next step without any purification.
If a pure product is required, it can be purified by column chromatography with the eluent being petroleum ether/ethyl acetate (3:1). Collect the effluent to obtain a white solid.
(3) Synthesis of 1H-1,2,3-triazole
Weigh 6.36 g (0.04 mol) of 1-benzyl-1,2,3-triazole in a 100 mL autoclave, add 40 mL of glacial acetic acid, add 0.6 g of 5% palladium on carbon, replace with hydrogen three times, and heat at 7 atm and 100 °C for 5 h.
Cool to room temperature, press the reaction solution out of the autoclave, filter, and evaporate the solvent under reduced pressure with a circulating water pump to obtain a light yellow oily substance. Vacuum distill with an oil pump and collect the fractions to obtain 2.41 g of colorless oily liquid with a yield of 87%.
Method 2
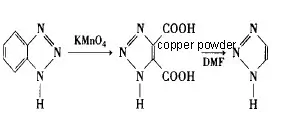
Benzotriazole was used as a raw material, potassium permanganate as an oxidant, and water as a solvent for oxidation at 105°C to obtain 1,2,3-triazole-4,5-dicarboxylic acid with a yield of 66.7%, and then microwave decarboxylation was used to obtain 1H-1,2,3-triazole with a total yield of 64.7%.
Operation steps
(1) Preparation of 1,2,3-triazole-4,5-dicarboxylic acid
Dissolve 6.7 g benzotriazole (0.0563 mol) in 200 mL water, heat to 105 °C, and slowly drop a saturated aqueous solution containing 50 g KMnO4 (0.317 mol), controlling the drop rate to keep the reaction solution at a constant boiling point.
After the dropwise addition is completed, reflux and monitor by TLC.
After the reaction is complete, add w(H2O2) = 30% hydrogen peroxide and excess KMnO4 to react until the purple color of the solution completely fades, and filter.
The filtrate is concentrated to 130 mL, and 30 mL of concentrated hydrochloric acid is added to the concentrate. Let it stand overnight to precipitate a white solid, filter it, and dry the resulting solid to obtain 5.8 g of the product 1,2,3-triazole-4,5-dicarboxylic acid (yield 66.7%).
(2) Synthesis of 1H-1,2,3-triazole
Add catalyst copper powder to a 50 mL N, N-dimethylformamide (DMF) solution containing 3.0 g 1,2,3-triazole-4,5-dicarboxylic acid (0.0191 mol), heat to 150°C, monitor by TLC, react for 6 h, cool to room temperature, filter, wash the filter cake with DMF three times, combine the DMF, and distill under reduced pressure to obtain 0.86 g (yield 65%) of colorless liquid, which is then frozen to obtain a white solid product 1H-1,2,3-triazole.
(2) Synthesis of 1H-1,2,3-triazole by microwave method
3.0 g 1H-1,2,3-triazole-4,5-dicarboxylic acid (0.0191 mol) was added to 50 mL DMF solution, and then copper powder was added as a catalyst. Then, the mixture was added to a microwave reactor and heated at 680 W for reaction. The reaction was monitored by TLC. After the reaction was complete (6 min), the mixture was treated according to Section 1.2.2 to obtain 1.28 g of product (yield 97%).
Method 3
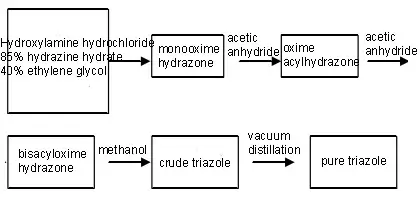
Use hydrazine hydrate, hydroxylamine hydrochloride and glyoxal as raw materials, and prepare them through oximation, hydrazone, deacetylation and other reactions.
Operation steps
(1) Synthesis of monooxime hydrazone
Reaction formula: C2H2O2 + NH2NH2·H2O + NH2OH·HCl → C2H5N3O
Reaction steps:
First, pour 23.5g of 85% hydrazine hydrate into a three-necked flask, and then slowly add 27.8g of hydroxylamine hydrochloride into the flask at about 0℃. Because heat will be released when hydroxylamine hydrochloride is added, the addition rate must be controlled so that the temperature in the flask does not exceed 5℃.
After adding hydroxylamine hydrochloride, continue to control the temperature in the kettle at about 0℃, add 58g of 40% glyoxal dropwise, and finally heat to 10-15℃ for 6 hours.
The obtained liquid after the reaction is extracted with ethyl acetate (1:1, v/v) 3 – 5 times to obtain an ethyl acetate solution of monooxime hydrazone, then the ethyl acetate solution is concentrated under reduced pressure, and then toluene is added to precipitate the monooxime hydrazone.
It was dried to obtain 19 g (yield 54.6%) for the next step of the reaction.
(2) Synthesis of oxime acylhydrazone
Reaction formula: C2H5N3O+C4H6O3→C4H7N3O2+CH3COOH
Reaction steps:
Put 19g of monooxime acylhydrazone obtained in the above reaction into a three-necked flask, add 76g of ethyl acetate, wait for it to dissolve, and filter out a small amount of insoluble matter.
Put the filtrate back into the flask, control the temperature in the kettle below 30℃, add 20.3g of acetic anhydride dropwise, and after the addition is complete, continue to maintain the temperature at 25-30℃ for 5 hours, then filter out the oxime acylhydrazone for use, and dry it to obtain 18.6g (yield 66%).
(3) Synthesis of bisacyl oxime hydrazone
Reaction formula: C4H7N3O2+C4H6O3→C4H5N3O
Reaction steps:
Put 18.6g of oxime acylhydrazone into a three-necked flask, add 55.8g of ethyl acetate and stir, control the temperature at about 90℃, add 14.7g of acetic anhydride dropwise, and after the addition is complete, control the temperature at 90℃ to react for 3 hours, then cool to room temperature, filter, and set the filtrate aside.
(4) Synthesis of triazole
Reaction formula: C4H5N3O+CH3OH→C2H3N3+CH3COOCH3
Reaction steps:
Put the filtrate from the previous reaction into a three-necked flask equipped with a stirrer, and add 44.6g of anhydrous methanol dropwise using a dropping funnel. The dropping temperature does not exceed 10°C. After the addition is completed, continue to react at 10°C for 4.5 hours. The obtained reaction solution is distilled under reduced pressure to obtain 1H-1,2,3-triazole.
Method 4
Use p-toluenesulfonyl hydrazide as a raw material to obtain 1H-1,2,3-triazole in one step through a cyclization reaction.
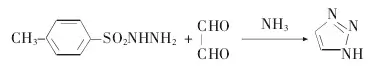
Operation steps
(1) Preparation of p-toluenesulfonyl hydrazide
Add 60g p-toluenesulfonyl chloride and 110 mL tetrahydrofuran to a 250 mL three-necked flask and stir to dissolve.
When cooled to 10℃ with ice water, 40ml 85% hydrazine hydrate was added dropwise. During the addition, the reaction temperature was controlled at 10-1510℃.
After the addition was completed, the reaction was continued for 15min.
The lower layer was removed with a separatory funnel, and the upper layer was washed twice with 20ml saturated sodium chloride solution, and then dried with anhydrous sodium sulfate.
Filter and wash the solid with 10ml tetrahydrofuran.
120 ml of petroleum ether was added to the filtrate under stirring and placed in a refrigerator overnight. A large amount of solid precipitated. The solid was collected by vacuum filtration. The solid was washed with 40 mL of petroleum ether and dried to obtain 56 g of solid.
(2) Preparation of 1H-1,2,3-triazole
160 ml of ethanol, 20 g (0.11 mol) of p-toluenesulfonyl hydrazide and 32 ml (0.28 mol) of glyoxal solution were added to the reaction flask, NH3 was introduced for 2 h, the reaction was continued for 20 h, the reaction was stopped, the solid was removed by vacuum filtration, the solvent was removed by rotary evaporation, and the fraction of 81-84/1.03 kPa was collected and solidified after freezing to obtain 5.4 g of white solid with a yield of 73%.
Method 5
(1) Preparation of benzotriazole:
It includes the following sub-steps:
1. In a reaction kettle, with ethanol as solvent, add a certain amount of o-nitrochlorobenzene and 3.5 times the molar ratio of o-nitrochlorobenzene to 85% hydrazine hydrate, heat to 80-110°C, reflux for 3-5h, and then add 20% NaOH solution, the amount of substance of the 20% NaOH solution is twice the amount of substance of o-nitrochlorobenzene.
2. The reaction liquid after the reaction in step 2 is under reduced pressure, the vacuum degree is controlled at 10-20kPa, the oil bath is heated, and the oil temperature is maintained at 75-80°C for distillation, and the initial fraction of about 10% is discarded, and then the hydrazine hydrate is collected and recovered; and the temperature is raised to 135-140°C to recover dimethyl sulfoxide.
3. Add a certain amount of water to the residual liquid after recovering hydrazine hydrate and dimethyl sulfoxide in step b until the residue is completely dissolved, then add 10% hydrochloric acid to adjust the pH to 3-3.5. At this time, a large amount of white crystals are precipitated, filtered and drained, and washed with ice brine 2-3 times to obtain white crystal 1-hydroxybenzotriazole.
4. Add 1-hydroxybenzotriazole and 2-3 times the molar amount of reduced iron powder relative to the molar amount of 1-hydroxybenzotriazole and 1-10 times the mass of water relative to the mass of 1-hydroxybenzotriazole into a reactor, heat to 85°C and keep warm for 1 hour, add 10% hydrochloric acid solution relative to the molar number of 1-hydroxybenzotriazole 1-2 times, the dropping time of the 10% hydrochloric acid solution is less than 1 hour, reflux and keep warm for 1-3 hours after the dropwise addition is completed, filter the excess iron powder while hot, add an appropriate amount of NaOH solution to the filtrate to adjust the pH of the filtrate to 3-4, then add an appropriate amount of saturated NaCl solution, extract with ethyl acetate, obtain the organic phase, distill and recover the solvent, crystallize and filter, and vacuum dry to obtain the product benzotriazole.
(2) Preparation of 1H-1,2,3-triazole using benzotriazole obtained in step (1)
Includes the following sub-steps:
1. Preparation of 1H-1,2,3-triazole-4,5-dicarboxylic acid
Add benzotriazole to water, heat to 100-105°C, keep warm for 1 hour, add potassium permanganate, and add potassium for 0.5-1 hour. After potassium permanganate is added, the reflux reaction is complete.
Keep warm at 90°C to 100°C for 2 hours, then drop 25-50% hydrogen peroxide and excess potassium permanganate solution to react until the purple color of the solution completely fades, filter, concentrate the filtrate, cool to room temperature, adjust the pH of the filtrate to 2-3 with hydrochloric acid, stir for half an hour, filter, wash the filter cake with a small amount of water, and dry at 80°C to 90°C for 4 hours.
Add the dried filter cake to 3-4 times the amount of methanol or ethanol, heat and reflux for half an hour, cool to room temperature, filter, and dry at 80℃-90℃ for 3 hours to obtain 1H-1,2,3-triazole-4,5-dicarboxylic acid.
2. Preparation of 1H-1,2,3-triazole crude oil:
Preparation of 1H-1,2,3-triazole: Dissolve the 1H-1,2,3-triazole-4,5-dicarboxylic acid obtained in step e in N, N-dimethylformamide solution, add a catalytic amount of copper powder, and then add it to a microwave reactor and heat it at 680W for reaction. After 6-10 minutes of reaction, obtain 1H-1,2,3-triazole crude oil.
3. Refining of crude 1H-1,2,3-triazole oil:
Add a catalytic amount of Lewis acid to the crude 1H-1,2,3-triazole oil obtained in step 2, raise the temperature to 80℃~100℃, slowly drop 25~50% hydrogen peroxide or potassium permanganate, add a small amount of activated carbon after the addition is complete, keep the temperature at 80℃~100℃ for 4 hours, filter to remove the activated carbon, transfer the filtrate to a container with a distillation column for vacuum distillation, collect the colorless and transparent fraction, and finally obtain the finished 1H-1,2,3-triazole.
Method 6
SnC12 reduction of azobenzene
The most commonly used method for the synthesis of 1H-1,2,3-triazole is the SnC12 reduction of azobenzene. It is achieved by reducing azobenzene with sodium hydrogen manganate.
The following are its detailed steps:
1. Add about 10 grams of azobenzene to 2.5 L of water.
2. Add 6.35 grams of sodium hydrogen manganate and stir evenly.
3. Slowly add 15mL of concentrated sodium hydroxide solution (30%).
4. Heat to a water temperature not exceeding 90℃, and slowly drip 50mL of SnC12 solution during heating. Control the reaction temperature between 75-80℃, and the reaction duration is 2-3 hours.
5. Cool to room temperature and adjust the pH to about 8 with sodium hydroxide.
6. Extract the product with acetic anhydride or chloroform, and then wash with NaHCO3 solution. 7. Use concentrated hydrochloric acid to adjust the pH value to acidic, distill and replace with an appropriate amount of acid or bromine to obtain the target product.
The advantages of this method are low cost, easy operation, and good synthesis effect. However, this method takes a long time, requiring 2 to 3 hours for reaction, and the purity of the product is not high.
Method 7
Stepwise method for the synthesis of 1H-1,2,3-triazole
The steps are as follows:
1. Bromination: First, mix 4-nitropyridine (10 g) and potassium ferrocyanide (24 g), then add the mixture to 100 mL of tetrahydrofuran (THF) and add 5 g of thiophenol.
The reaction needs to be cooled, and the liquid color will turn dark orange.
2. Cyclization: The compound obtained in the previous step is converted into tetrahydrofuran in tetrahydrofuran.
Sodium hydride needs to be added at this stage; the reactant temperature is room temperature, and the reaction time is 5 hours.
3. Ring-opening: The reaction mixture is acidified to pH 3 with an excess of HCl aqueous solution, and the obtained 1H-1,2,3-triazole product can be obtained by oxidation reaction to obtain the target product.
The advantage of the stepwise method is that the product purity is higher, but more steps are required and the reaction time is also longer.
Method 8
Acyloxy Synchronous Alkylation Synthesis
Acyloxy Synchronous Alkylation is also a commonly used method for synthesizing _1H-1,2,3-triazole.
The steps are as follows:.
1. First, dissolve ferrous sulfate (3 g) in 1,4-dioxobutyric acid (10 g). Stir at a low temperature for 15 minutes.
2. Slowly drip tetramethyl chemosilicon detergent (TMCS) into the reaction mixture at a volume ratio of 0.5: 1 and stir in an ice bath for two hours.
3. The higher temperature is equivalent to the E1cB reaction, because the acyloxy and alkyl groups in the E1cB reaction are simultaneously deionized to produce intermediates, and finally the target product is formed under the action of acid.
The advantage of this method is that the reaction is simple and convenient, but multiple reaction incubation processes are required, and the corresponding yield will be reduced. In addition, the technology to improve purity also requires some additional arrangements.
Finally
The above are 8 preparation/production methods of 1H-1,2,3-triazole.
ORIENTRED is a professional manufacturer and supplier of 1H-1,2,3-triazole.
If you want to buy 1H-1,2,3-triazole or want to know more about 1H-1,2,3-triazole, please feel free to contact us.

About Author
20 years of experience in the research and development of pharmaceutical intermediates and APIs.
Committed to providing you with correct and effective solutions with professional knowledge.
Looking forward to establishing a long-term cooperative relationship with you.
If you have any needs, please feel free to contact us.