N,N′-Carbonyldiimidazole Basic Information, Physicochemical Properties, etc.
| Basic Information |
| Name |
1,1′-Carbonyldiimidazole |
| Synonyms |
carbonyldiimidazole; carbodiimidazole; di(1h-imidazol-1-yl)methanone;cdi;(diimidazol-1-yl)ketone; N,N′-Carbonyldiimidazole; 1H-Imidazole, 1,1′-carbonylbis- |
| CAS Number |
530-62-1 |
| Molecular Formula |
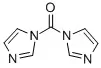
C7H6N4O |
| Molecular Weight |
162.15 |
| EINECS Number |
208-488-9 |
| Structural Formula |
 |
| Physicochemical Properties |
| Melting Point |
117-122 °C(lit.) |
| Boiling Point |
288.83°C (rough estimate) |
| Density |
1.303 g/mL at 25 °C |
| Refractive Index |
n20/D1.434 |
| Solubility |
Soluble in dimethylformamide. |
| Water Solubility |
Insoluble in water |
| Form |
Crystalline powder |
| Color |
White to light cream |
| Stability |
Stable but sensitive to moisture. Incompatible with acids, strong oxidants and water. |
| Acidity Coefficient (pKa) |
2.90±0.10(Predicted) |
| Safety and Regulatory Information |
| Merck |
14,1819 |
| BRN |
6826 |
| NIST Chemical Substance Information |
|
| EPA Chemical Substance Information |
N,N′-Carbonyldiimidazole (530-62-1) |
| Other Information |
| Detection Methods |
|
1,1′-Carbonyldiimidazole Application, Use, Usage, Synthesis Method, etc.
Main application areas and uses
Basic properties
- High reactivity, capable of reacting with functional groups such as -COOH, -NH₂, and -OH.
- Can synthesize compounds that are difficult to prepare by conventional methods, such as ketones, esters, and ureas.
- Replace highly toxic phosgene (such as the synthesis of imidazole pesticides) with safer.
Medicine and biotechnology
- Key reagents for protein and peptide synthesis, keeping the molecular configuration unchanged.
- Used for enzyme immobilization, antibiotic and drug intermediate synthesis.
- Synthesis of triphosphate nucleosides, peptides, and ester condensation agents.
Organic synthesis
- Efficient activation of carboxylic acids, simpler and cheaper than DCC and EDC.
- Synthesis of amides, ureas, β-keto sulfones, β-ene amino acid derivatives, etc.
- Coupling reagents for nonlinear optical materials (such as dipole polyamides).
Pesticides and antibacterial agents
- Synthesis of imidazole pesticides (avoid the use of phosgene).
- Preparation of antibacterial amphiphilic urea oligomers.
New materials and heterocyclic compounds
- Synthesis of self-assembled hydroxyquinoline derivatives and heterocyclic compounds.
- As a carbonyl conversion reagent, it is used in the synthesis of new heterocyclic compounds.
Preparation method
Phosgene method
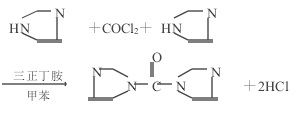
In a four-mouth reaction bottle with a stirrer, a thermometer, and a gas guide tube, add toluene, tri-n-butylamine and imidazole, and heat to 70°C.
After introducing phosgene within 20 to 30 minutes, maintain the reaction temperature, react for 1.5 hours, cool to 20°C, and react for 45 minutes.
Filter and separate the white crystalline precipitate of imidazole hydrochloride, concentrate the filtrate, wash with toluene, and dry under ammonia purge to obtain 1,1′-carbonyldiimidazole with a yield of 91%.
Caution! Phosgene is toxic, and this operation should be performed in a fume hood.
Carbonate method
(1) Dissolve imidazole in hexamethyldisilazane and add potassium carbonate. Stir and react at 100-110°C for 5-6h, cool to 45-70°C and distill under reduced pressure. Collect the fraction at 75-78°C/1.03kPa (i.e., collect the fraction at 75-78°C under 1.03kPa pressure) to obtain N-trimethylsilyl imidazole.
(2) Dissolve diphenyl carbonate in xylene and pass nitrogen gas to protect. Add aluminum chloride, sodium methoxide and N-trimethylsilyl imidazole obtained in step (1). React at 140-150°C for 4-5h to obtain the carbonyl diimidazole reaction solution.
(3) Cool the carbonyl diimidazole reaction solution obtained in step (3) to 50-60°C, concentrate under reduced pressure, filter and vacuum dry the solid part to obtain carbonyl diimidazole.
Carbonyldiimidazole Packaging, Storage, Delivery, Transportation, etc.
Packing: 25kg fiber drum or Customer specified packaging
Storage:
- Store in a cool, dry, well-ventilated warehouse.
- Keep away from fire and heat sources.
- Avoid direct sunlight.
- Package tightly.
- Store separately from acids and edible chemicals and avoid mixing.
- The storage area should be equipped with appropriate materials to contain leaks.
Delivery: delivery within 3- 5 working days
Transportation: According to your needs
FAQ About Carbonyldiimidazole
Where can I buy 1,1′-Carbonyldiimidazole? What is the minimum order quantity? How much is it?
Answer: You can buy it through ORIENTRED, there is no minimum order quantity limit. Contact us at any time to get the latest quote.
Why must I use N,N′-Carbonyldiimidazole?
Answer: Other condensation agents may be cheaper or safer, but in some key reactions, the effect and purity of CDI are irreplaceable.