1-Indanone | CAS: 83-33-0
| Name | 1-Indanone; 2,3-Dihydro-1H-inden-1-one |
| CAS | 83-33-0 |
| Appearance | Pale yellow flake crystals |
| Molecular Formula | C9H8O |
| Molecular Weight | 132.16 |
| Assay | 99.5% |
| Packing, Delivery | 25kg/drum; Delivery within 3-5 working days |
| Sample | Available |
| Payment | L/C, D/A, D/P, T/T, Other |
ORIENTRED is a leading manufacturer and supplier of 1-Indanone (CAS: 83-33-0) in China, focusing on producing 1-Indanone with high purity/assay.
- 1-Indanone is of good quality and cheap price, and is produced in strict compliance with ISO9001, cGMP and other standards.
- We provide free samples, trial orders, bulk purchases of tons of 1-Indanone, procurement tiered pricing, and cost reduction 15%+.
- Open third-party testing and fast logistics services
Contact us now to get your exclusive quotation and COA sample.
1-Indanone Basic Information, Physicochemical Properties, etc.
| Basic Information | |
| Name | 1-Indanone |
| Synonyms | 4-fluorosulfonylbenzoicacid[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxy-2-oxolanyl]methylesterhydrochloride;A-HYDRINDONE;1-Indone;2,3-dihydro-1h-inden-1-on;2,3-Dihydro-1-indanone;2,3-Dihydro-1-indenone;alpha-Indanone;Dihydro-1-indenone |
| CAS Number | 83-33-0 |
| Molecular Formula | C9H8O |
| Molecular Weight | 132.16 |
| EINECS Number | 201-470-1 |
| Structural Formula | 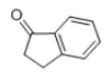 It contains an aromatic ring and a keto group (C=O). In the molecular structure of 1-indanone, a carbon atom on the aromatic ring forms a double bond with an oxygen atom to form a keto group. It contains an aromatic ring and a keto group (C=O). In the molecular structure of 1-indanone, a carbon atom on the aromatic ring forms a double bond with an oxygen atom to form a keto group. |
| Physicochemical Properties | |
| Melting Point | 38-40 °C(lit.) |
| Boiling Point | 243-245 °C(lit.) |
| Density | 1.103 g/mL at 25 °C(lit.) |
| Refractive Index | 1.5610 (estimate) |
| Solubility | The solubility in water is 6.5 g/L |
| Water Solubility | 6.5 g/L (20 C) |
| Form | Crystal |
| Color | Light yellow to brown |
| Stability | Photosensitive |
| Odor Threshold | 0.0088ppm |
| Safety and Regulatory Information | |
| Merck | |
| BRN | 507957 |
| NIST Chemical Substance Information | 2,3-Dihydro-1H-inden-1-one (83-33-0) |
| EPA Chemical Substance Information | 2,3-Dihydro-1H-inden-1-one (83-33-0) |
| Other Information | |
| Odor | A fairly light woody and somewhat medicinal scent with incense-like undertones. |
1-Indanone Application, Use, Usage, Synthesis Method, etc.
Core application areas of 1-indanone
Pharmaceutical active molecules and natural products
As a synthetic precursor, it is widely used in the development of new drugs, for example:
- Anti-Alzheimer’s disease (AD) drugs: acetylcholinesterase inhibitors (such as donepezil, approved by the FDA).
- Anti-Parkinson’s disease drugs: key intermediates for the synthesis of rasagiline (already available in the United States and Europe).
Anti-tumor research
1-Indanone derivatives (such as indanocine) have selective cytotoxicity, can induce apoptosis of drug-resistant cancer cells, and are harmless to normal cells, and have significant potential in tumor treatment.
Antibacterial and antiviral
Some derivatives show antibacterial and antiviral activities and can be further optimized as lead compounds.
Other biological activities
Polyphenol derivatives (such as paucifloral F) have anti-osteoporosis activity.
Natural extracts (such as 1-methoxy-6-methyl-3-oxo-2,3-dihydro-1H-indene-4-carbaldehyde in seaweed) contain 1-indanone structure and have potential medicinal value.
As an important intermediate for the synthesis of Indinavir.
1-Indanone Synthesis Method
Pass dry hydrogen chloride into freshly distilled indene, keep the temperature at 5-10℃, the reaction takes 8-10h, the reaction product is distilled under reduced pressure, and 90-103 (1.73kPa) is collected to obtain α-chlorodihydroindene.
Oxidize it with chromium trioxide in glacial acetic acid, keep the temperature at 35-40℃, and dihydro-1-indanone is generated.
The yield is 50%-60%.
Introduction to other synthesis methods
1. Polyphosphoric acid (PPA) cyclization method
This method uses a wide range of raw materials. Polyphosphoric acid (PPA) is used as a catalyst and solvent, and unsaturated acid is used as an acylating agent to synthesize indanone.
The reaction is relatively mild, avoiding dangerous operations such as high temperature and high pressure, reducing the risk of synthesis, and has the potential for large-scale production, but it produces a lot of waste acid, which has a greater impact on the environment.
For example, veratraldehyde and malonic acid are used as raw materials, and Knoevenagel reaction and selective reduction are carried out, and finally PPA cyclization method is used to obtain 5,6-dimethoxy-1-indanone.
2. Nazarov cyclization method
Nazarov cyclization reaction, also known as Nazarov cyclization reaction, refers to a type of chemical reaction in which divinyl ketone compounds undergo rearrangement reaction under the action of proton acid or Lewis acid to produce cyclopentene derivatives.
In 2007, Zhu Hongwei used copper trifluoroacetate as a catalyst to catalyze the tandem Nazarov cyclization electrophilic fluorination reaction to stereoselectively synthesize fluorinated 1-indanone derivatives, and studied the method of synthesizing fluorinated indanone derivatives. The experiment found that copper trifluoroacetate was the catalyst and NFSI was the fluorinating agent.
3. Ester condensation method
5-chloro-2-methoxycarbonyl-1-indanone is the raw material of (s)-5-chloro-2-methoxycarbonyl-2-hydroxy-1-indanone, an important intermediate in the synthesis of the insecticide indoxacarb.
The insecticide indoxacarb is only biologically active when it is the s-isomer, and the synthesis of (s)-5-chloro-2-methoxycarbonyl-2-hydroxy-1-indanone is the key step in the total synthesis of indoxacarb.
5-Chloro-2-methoxycarbonyl-1-indanone was obtained as a raw material by Annis G D et al. in 2001 through an ester condensation reaction.
4. Intramolecular Friedel-Crafts cyclization method
This method refers to the acylation reaction of aromatic hydrocarbons in which the hydrogen atoms on the aromatic ring are replaced by alkyl or acyl groups under the catalysis of Lewis acids such as anhydrous AlCl3, ZnCl2, or FeCl3. It is widely used in the preparation of organic intermediates such as aromatic ketones.
In 2008, Dai Liyan et al. studied the Friedel-Crafts cyclization method for the synthesis of 6-fluoro-2-methylindanone.
This intermediate is an important intermediate of the non-steroidal anti-inflammatory drug sulindac. It is obtained by condensation of p-fluorobenzyl chloride and diethyl methylmalonate, followed by alkaline hydrolysis, decarboxylation, SOCl2 chlorination, and intramolecular Friedel-Crafts acylation reaction. The total yield of the reaction is 57.0%.
5. Photochemical method
This method is induced by light. After the photolysis product absorbs photons, it becomes a singlet excited state and then generates a triplet state through intersystem crossing. Then, intramolecular hydrogen transfer occurs, and it becomes an enol form of Z/E configuration. The enol form of E configuration undergoes rearrangement and an intramolecular rearrangement reaction to obtain a substituted indanone.
This synthesis method has the advantages of mild reaction, simple method, rapid reaction, relatively high photoreaction yield, relatively easy separation and purification of products, and green environmental protection. However, a monochromatic light source is required in the reaction, which hinders the industrial application of this method.
6. Pinacol rearrangement method
Pinacol rearrangement refers to a special intramolecular rearrangement of a substituted vicinal diol catalyzed by Lewis acid or dilute sulfuric acid, etc., heating to remove a portion of water, and generating a reaction of aldehyde and ketone. The vicinal diol with a hydroxyl group on the ester ring is pinacol, and the ketone generated by the rearrangement is called pinacolone.
2,3-Dihydro-1H-inden-1-one Packaging, Storage, Delivery, Transportation, etc.
Packing: 25kg /drum or Customer specified packaging
Storage: Stored in a cool, dry, and ventilated place out of light
Delivery: delivery within 3- 5 working days
Transportation: According to your needs
FAQ About 2,3-Dihydro-1H-inden-1-one
Where can I buy 1-Indanone? Price and minimum order quantity?
Answer:
Purchase through chemical suppliers such as Sigma-Aldrich, TCI, ORIENTRED.
We have no minimum order quantity limit, Please email us to get the latest quote.
Are there any special conditions for transportation?
Answer:
Sealed transportation at room temperature, no dangerous goods certificate is required, but light-proof and moisture-proof packaging is required.
What is 1-Indanone?
Answer:
1-Indanone is a biochemical reagent that can be used as a biomaterial or organic compound for life science-related research.
1-Indanone is also an oxidation product and a component of fuels, solvents, and varnishes. It is part of the steroid hormone biosynthesis and arachidonic acid metabolic pathways.






